 Marlin Steel often makes custom wire mesh baskets for ultrasonic parts cleaning or other washing processes. However, these aren’t the only uses for mesh baskets. Recently, a company specializing in processing solutions for petrochemicals, hydrogen and gas fuel, and water reached out to Marlin to commission a basket to use in a catalytic reaction during a specific refinement process—one involving hydrogen sulfide and high temperatures.
Marlin Steel often makes custom wire mesh baskets for ultrasonic parts cleaning or other washing processes. However, these aren’t the only uses for mesh baskets. Recently, a company specializing in processing solutions for petrochemicals, hydrogen and gas fuel, and water reached out to Marlin to commission a basket to use in a catalytic reaction during a specific refinement process—one involving hydrogen sulfide and high temperatures.
Key Challenges in This Design
This particular design posed a few specific challenges for the Marlin Steel team:
First, the wire mesh needed to be incredibly tight—the open space between wires was only 0.03 inches—to keep particulates from slipping between chambers while still allowing the catalyzing fluid to seep into and out of the basket. Why? Because, the client couldn’t allow their particle catalyst to drift from one chamber to another—let alone have it fall out of the basket.
Second, the basket had to be resistant to contamination from sulfur-rich environments over long periods of time. Sulfur is not only a common component of petroleum and natural gas extraction, but H2S (hydrogen sulfide) was also a key component of the refinement process the basket would be used in. This particular substance is considered dangerous by OSHA. High concentrations of H2S can cause those exposed to suffer symptoms like: “shock, convulsions, [unablility] to breathe, coma, death; effects can be extremely rapid (within a few breaths).”
H2S is known to cause corrosion in steel—and can even cause iron particles to be lost as, according to research articles from the Ohio University Department of Chemical Engineering, “the corrosion rate is always higher than the scale retention rate. The scaling tendency under the test conditions indicates that between 40% and 72% of the iron consumed by corrosion ended up as iron sulfide on the steel surface, with the balance lost to the solution.” In short, between 28 and 60% of the steel’s iron content can be lost completely to the chemical reaction with H2S—and that’s just after an hour of exposure.
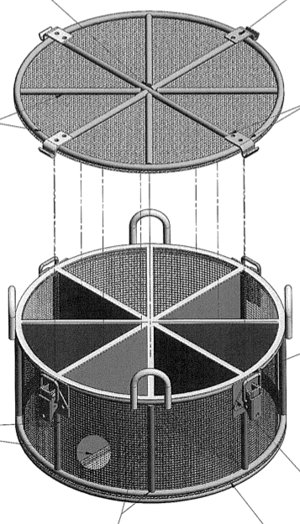
Also, the client needed to process multiple catalysts at once to ensure process efficiency. However, the catalysts couldn’t be allowed to come into contact with one another, so the basket needed to have eight separate chambers.
Finally, the process temperature at the finishing point was a concern as well. At the end of the catalyzing process, temperatures would typically exceed 800°F—well above the melting point of most polymers. This, paired with the risk of webbing in such finely-spaced wire mesh, eliminated the possibility of using a polymer coating to protect the basket from the hydrogen sulfide exposure.
Meeting the Challenge
In this application, meeting the requirements for open space was the easiest challenge to tackle. By using advanced factory automation to assemble baskets, the 0.03” open space requirement was easy to meet.
However, making sure the baskets could last for more than a couple of uses proved a bit trickier. The temperatures involved in the process eliminated using polymer coatings as an option, so the base material of the wire basket would have to be able to resist H2S exposure on its own.
Here, the use of grade 316 stainless steel was determined to be the best option. 316 SS would be able to withstand the chemicals involved in the process with less risk of scaling than grade 304 SS. This would keep the basket in better shape for longer periods of time—extending its useful life and making it so that the client wouldn't have to constantly reorder it over and over again.
To prevent particulates from floating from one chamber of the basket to the next, not only were the dividers between chambers made of very fine wire mesh, but that mesh was also permanently welded to the sides of the basket to eliminate gaps.
A latched lid was added to the design to prevent catalyst particles from floating out of the basket. Four “handles” were added to the sides of the round wire basket for easy handling and for attaching hooks so the basket could be suspended in a reactor. The reason these handles were put onto the frame instead of adding a hoist hook to the lid was to eliminate risk of the lid deforming over time and creating a gap that could let catalyst particles float from one basket chamber to another.
As with all custom wire baskets, the design was passed through a finite element analysis (FEA) and checked for flaws over and over again. Any flaws or failures uncovered during this analysis would be studied—and then used to modify the design.
Thanks to this analytical process, Marlin’s degreed engineers were able to create the perfect basket to meet the client’s needs without needing to make physical prototypes. This saved a considerable amount of time by letting Marlin run thousands of different tests in hours rather than waiting weeks or months on physical trials.
The end result was that Marlin’s team was able to deliver a high-quality basket to the client on short notice—it only took a couple of weeks to go from initial design to final delivery. This fast turnaround helped the client meet their timetables and stay on track for their refinement processes.


.gif)


